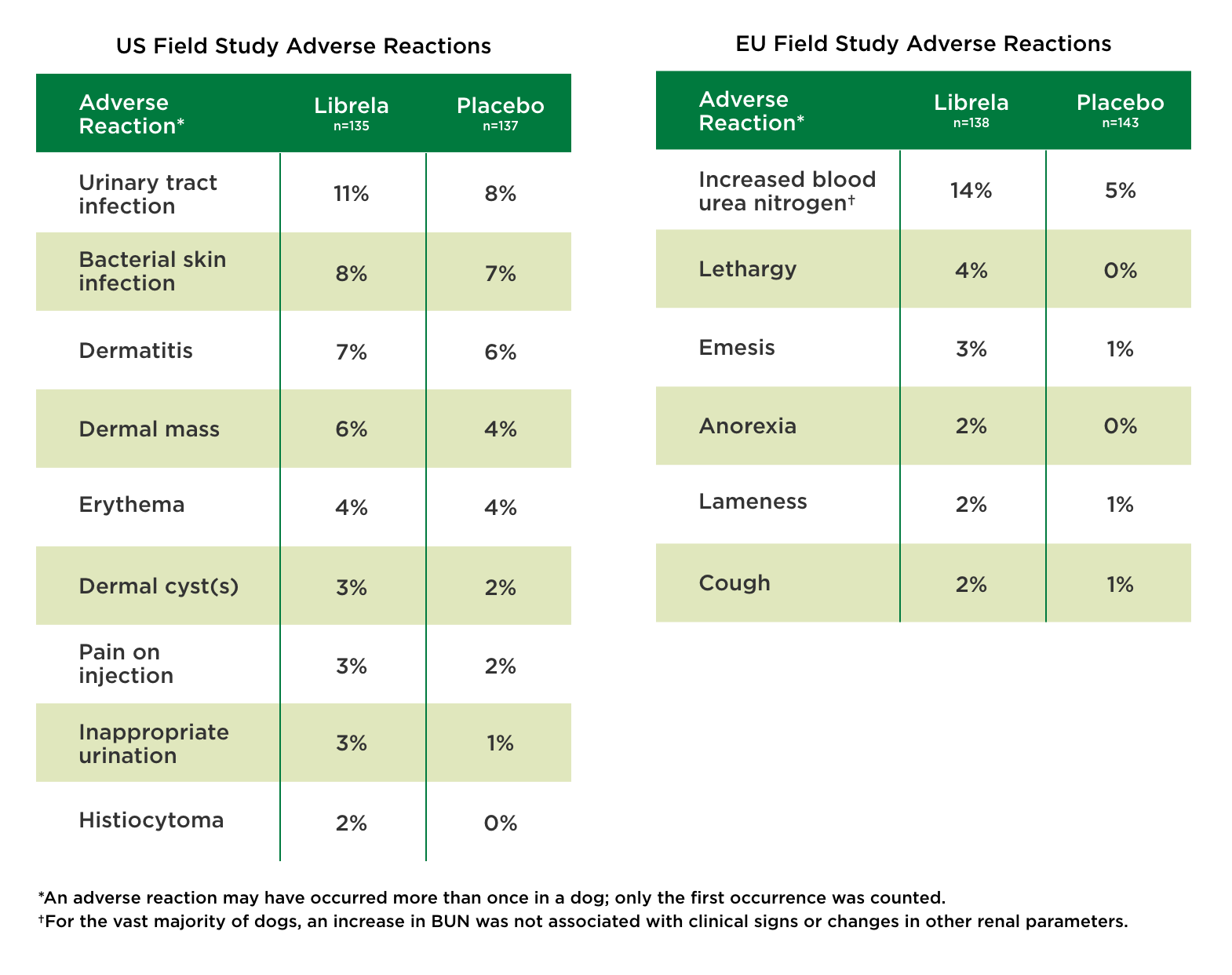
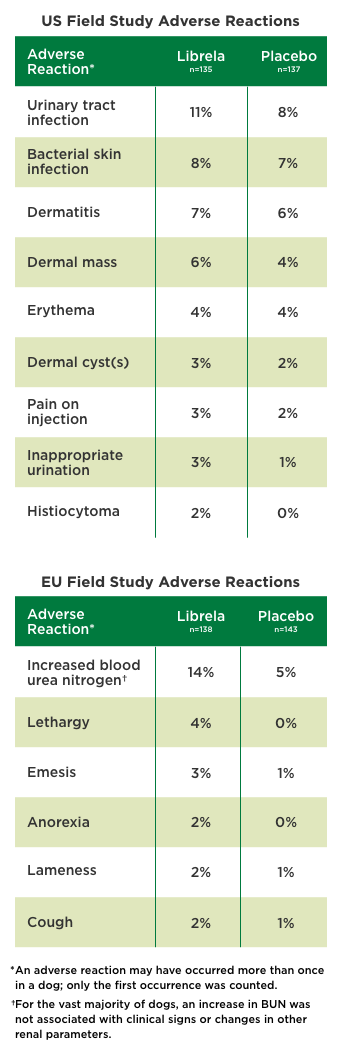
Dosing
The recommended dose of Librela is 0.23 mg/lb (0.5 mg/kg) body weight.
![]()
For dogs <11 lb (<5 kg): Aseptically withdraw 0.045 mL/lb (0.1 mL/kg) from a 5 mg/mL vial into a single syringe and administer immediately. Discard the vial after the dose has been withdrawn.
![]()
For dogs ≥11 lb (≥5 kg): Dogs should be dosed by weight range according to the Librela dosing chart. Dogs are given the full content of 1 or 2 vials of the appropriate concentration based on body weight. Aseptically withdraw the total dose into a single syringe and administer immediately.